Using the RefillRx mobile app? Then you will love our new, ENHANCED Sentry Drug Center mobile app.
Quickly request refills or login and manage your prescriptions on the go!
Available on both iTunes and Google Play.
Call or Visit for All of your Vaccination Needs!
Get Healthy!
Results for search "Drug Approvals".
Health News Results - 20
Pharmaceutical companies are using the citizens of lower-income countries as guinea pigs to test cutting-edge drugs headed mainly for the United States and other well-off nations, a new study says.
Only a quarter of medicines tested in other countries wound up available to the citizens there within five years of the drugs’ approval by the U.S. Food and Drug Administration (FDA), res...
- Dennis Thompson HealthDay Reporter
- |
- November 18, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) has appointed one of its most respected cancer drug regulators to lead the agency’s main division for approving new drugs.
The appointment of Dr. Richard Pazdur comes after a turbulent year with hundreds of staff departures within the agency.
Pazdur, who has ...
- I. Edwards HealthDay Reporter
- |
- November 13, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) has approved a new nonhormonal treatment to help women manage menopause symptoms such as hot flashes and night sweats.
Elinzanetant (Lynkuet), a once-daily pill, is expected to be available within weeks.
These uncomf...
- Deanna Neff HealthDay Reporter
- |
- October 27, 2025
- |
- Full Page
Drugmaker Eli Lilly plans to buy Verve Therapeutics, a gene-editing startup, for about $1 billion upfront.
The deal gives Lilly a potential new treatment for heart disease, The Wall Street Journal reported.
The deal, announced June 17, includes a cash offer of $10.50 per share for all outstand...
- HealthDay Reporter
- I. Edwards
- |
- June 18, 2025
- |
- Full Page
Texas has moved to fund research into ibogaine, a psychedelic drug that may help treat addiction, depression and brain injuries.
Gov. Greg Abbott signed a bill last week approving $50 million in state funds for ibogaine research, The New York Times reported.
The goal is to support clinic...
- HealthDay Reporter
- I. Edwards
- |
- June 16, 2025
- |
- Full Page
The U.S. Food and Drug Administration (FDA) has approved a new drug for a serious heart condition that affects thousands of people.
The drug, called Amvuttra (vutrisiran), is made by Alnylam Pharmaceut...
- HealthDay Reporter
- I. Edwards
- |
- March 24, 2025
- |
- Full Page
Another experimental drug meant to slow the damage of Alzheimer's appears poised to join a growing arsenal of new treatments for this memory-robbing disease.
In research published online Monday in the Journal of the American Medical Association and presented simultaneously at the Alzheimer's A...
- HealthDay Reporter
- Robin Foster
- |
- July 17, 2023
- |
- Full Page
The U.S. Food and Drug Administration on Thursday gave full approval to the Alzheimer's drug Leqembi, clearing the way for insurance coverage of the pricey drug.
"The full FDA approval will open the floodgates for people with early Alzheimer's to get this drug. It's a big deal because it's very expensive at $26,500 per year,"
The U.S. Food and Drug Administration on Tuesday approved two drugs that have been used in adults with type 2 diabetes for years for use in children aged 10 and up.
The approvals of Jardiance (empagliflozin) and Synjardy (empagliflozin and metformin hydrochloride) provide a new class of medications for pediatric type 2 diabetes. They join metformin, which has been approved for children wi...
- HealthDay Reporter
- Cara Murez
- |
- June 21, 2023
- |
- Full Page
Patients with Crohn's disease have a new treatment option, following U.S. Food and Drug Administration approval of a pill called Rinvoq (upadacitinib).
Rinvoq is meant to treat adults with moderately to severely active Crohn's disease who have not had success with TNF (tumor necrosis factor) blockers. The daily pill is the first oral treatment for this group of patients.
Crohn's is ...
- HealthDay Reporter
- Cara Murez
- |
- May 19, 2023
- |
- Full Page
A medication to treat agitation in Alzheimer's patients now has approval from the U.S. Food and Drug Administration.
The FDA gave supplemental approval to Otsuka Pharmaceutical Company Ltd., and Lundbeck Inc. for Rexulti (brexpiprazole) oral tablets on Thursday. Rexulti is the first FDA-approved treatment for these symptoms.
"Agitation is one of the most common and challenging aspec...
- HealthDay Reporter
- Cara Murez
- |
- May 11, 2023
- |
- Full Page
Another experimental drug meant for Alzheimer's disease looks so promising that drugmaker Eli Lilly plans to ask the U.S. Food and Drug Administration for full approval by the end of June.
Known as donanemab, the medication clears amyloid plaque from the brain. In a late trial, the drug slowed memory and thinking declines in early symptomatic Alzheimer's patients by more than a third, Li...
- HealthDay Reporter
- Cara Murez
- |
- May 3, 2023
- |
- Full Page
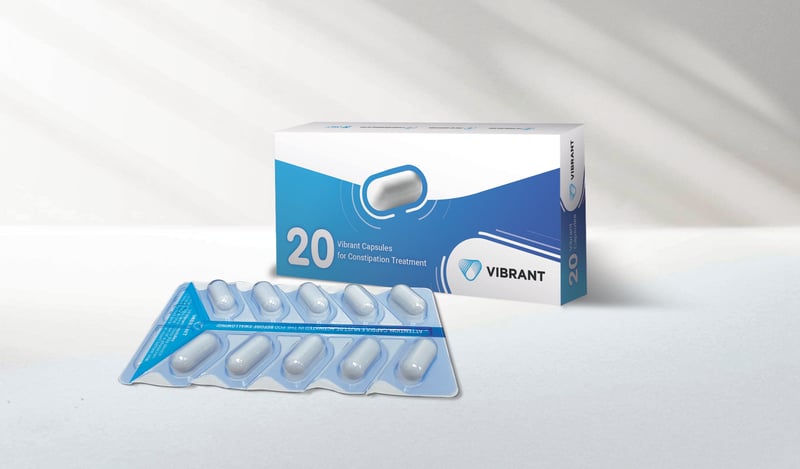
A new treatment for chronic constipation may bring relief without having to use drugs.
It's a vibrating pill called Vibrant that stimulates the colon as it passes through the body.
Although the pill was
Adults with asthma now have a new rescue medication to turn to after the U.S. Food and Drug Administration approved Airsupra on Wednesday.
The drug is the first approved to combine albuterol (a beta-2 adrenergic agonist) and budesonide (a corticosteroid).
It's mea...
- HealthDay Reporter
- Cara Murez
- |
- January 12, 2023
- |
- Full Page
The U.S. Food and Drug Administration on Wednesday approved the first fecal microbiota treatment, aimed at helping adults battling tough-to-treat Clostridium difficile (C. diff) infections.
"Today's approval of Rebyota is an advance in caring for patients who have recurrent C. difficile infection [CDI]," said
People with one form of the genetic blood disorder hemophilia now have a one-time treatment with a $3.5 million price tag.
The U.S. Food and Drug Administration approved the new gene therapy Hemgenix on Nov. 22. Soon after, drugmaker CSL Behring revealed its cost.
The company said its drug would ultimately reduce health care costs because patients with the genetic disorder would ne...
- HealthDay Reporter
- Cara Murez
- |
- November 23, 2022
- |
- Full Page
People with a rare genetic form of ALS may benefit from extended use of an investigational drug, a new study shows.
The medication, tofersen, benefited patients with mutations of the gene SOD1. These mutations create a misfolded version of a protein, which leads to amyotrophic lateral sclerosis, also kn...
- HealthDay Reporter
- Cara Murez
- |
- September 23, 2022
- |
- Full Page
Merck's experimental COVID-19 antiviral pill appears effective, but may pose risks for pregnant women, including birth defects and toxicity to developing fetuses, according to the U.S. Food and Drug Administration.
On Friday morning Merck announced